


|
Nanjing Norman Biological Technology Co., Ltd
|
| Payment Terms: | T/T,L/C,D/A,D/P,WU,Paypal,Money Gram |
| Place of Origin: | Jiangsu, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
NGAL Rapid Test Poct Kits
Methodology
Fluorescence Immunoassay
NGAL
1.The optimal marker for early diagnosis of acute kidney injury(AKI).
2.Independent risk index to evaluate
3.progression of chronic kidney disease(CKD)
NGAL Urine Specs
Methodology | Fluorescence Immunoassay |
Specimen | urine |
Measuring range | 5-1500ng/ml |
Cut-off value | 5ng/ml |
Reaction time | 15 minutes |
Shelf life | 12months |
Clinical Significance
1.Optimal indicator for early diagnosis of acute kidney injury
2.Real-time risk stratification of acute kidney injury
3.Treatment and detection of acute kidney injury and prognosis evaluation
Performance Analysis
Correlation analysis between Norman NGAL and Abbott NGAL
Advantage
High sensitivity:5ng/ml
High accuracy: 98% correlation with Abbott
Wide linear range:5-300ng/ml
Small sample size:5ul(urine)
Applicable Instruments
Fluorescence Immunoassay Analyzer
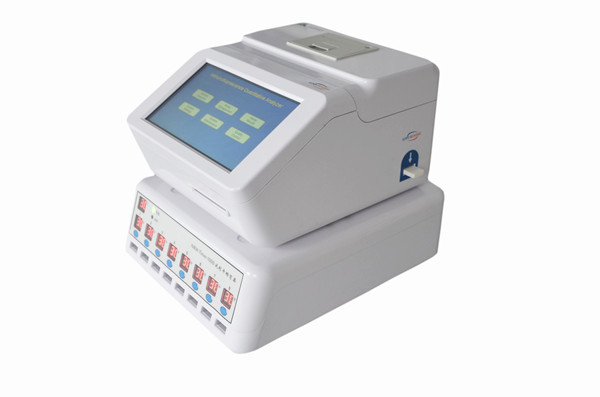
Fluorescent Immunoassays (FIA)
Fluorescent Immunoassays are simply a different type of immunoassay.
The key variable is the biochemical technique used for detecting the
binding of the "detection" antibody and the analyte molecule.
The advantages of a Fluorescent detection system
The advantages of a Fluorescent detection system have been known for many
years. These include higher sensitivity detection of the analyte, simplified
reagents and simpler assay designs.
Several breakthroughs have occurred over the past few years that have enabled
the implementation of a fluorescent based immunoassay system at the point of
care.
NGAL Kits Show
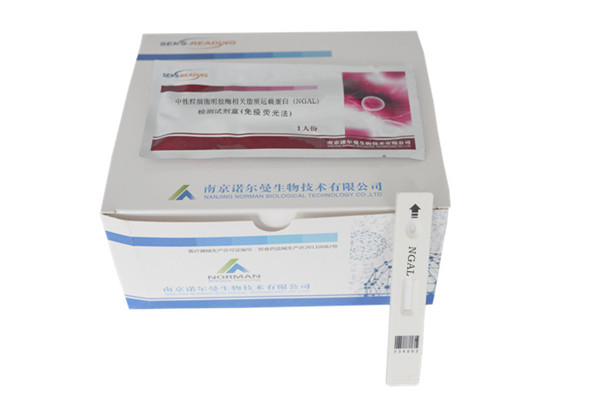

Product Name
Methodology:Fluorescence immunoassay
The optimal marker for early diagnosis of acute kidney injury(AKI).
Package
Test card: Aluminum foil pouch 1pc/bag, desiccant
Package size: 25pcs/box
Test Method
Immunefluorescence techniques
Instruction for use :
Please read the manual and instrument operation
carefully before using.Check whether the equipment can operate regularly,
and prepare other related materials.
Allow the test device, PCT buffer and specimen balanced to room temperature
(15-30℃) prior to testing.
Minimizing exposure to damp air.
Interpretation of test results
1 The analysis of test results are accomplished by immune-fluorescence quantitative
analyzer of our company's NORMAN series.
2 The concentration of NAGAL in the samples can be read directly in the corresponding
batch of ID card standard curve by fluorescence signal valuewe measured. The default
results use ng/ml as a unit.
Test Procedure
1.Open the Immune-fluorescence quantitative analyzer.
2.Check whether the batch number of IC card and reagent box is consistent, and then
insert IC card into the instrument to get the curve we wanted.
3.Remove the test device from the protective pouch and place it on a flat surface. Draw
in 5μl of urine sample to the centrifugal tube, and dilute with 90μl of diluents. After
gently mix the sample, add 90μl of diluted sample into the sample well.
Place the test strip at room temperature for 5 minutes.
4.Insert the test card into the hole of immune-fluorescence quantitative analyzer, and
click start, the instrument will scan the test card automatically.
5.Immune-fluorescence quantitative analyzer detects results automatically and calculates
the content of NGAL in the sample.
6.Specimens, used strip and transfer pipettes may be potentially infectious. Proper handling
and disposal methods should be established by the laboratory direction in accordance
with local regulations.
Package & Delivery


Related Products
About us
Nanjing Norman Biological Technology Co., Ltd is dedicated to R&D and
manufacturing of automated chemiluminescence system. Founded in 2008, Norman
biological has been upholding the idea that R&D shapes future ,and concentration
determines success. Ever since the beginning, Norman has been focusing on developing
and manufacturing chemiluminescence instruments and reagents.
Norman's manufacture center is located in the Yuhua District and owns an over
2,000m2 GMP-approved clean workshop. The R&D base, which is over 2,000m2 ,
is located in state-level new Jiangbei district. Now there are over 100 R&D engineers,
40% of which hold a PhD or master's degree.

Powered by advanced technology and excellent talents in the IVD field, Norman has been
consistently improving its innovation platform , and increasing R&D investment. Self-
innovation, combined with long-term strategic cooperation with universities and research
institutes and with outsourced technologies, ensures consistent improvement on product
quality.
Norman's R&D field has covered instruments, reagents, and raw materials, and has been
entrusted by the Nanjing government to build a R&D center specialized in biological
chemistry and immunity diagnosis. Up to now, Norman has acquired over 20 patents.
Being an expert in automated chemiluminescence analysis , Norman owns independent
and completed intellectual property rights, and its products providetop-notch sensitivity,
precision and accuracy . Thanks to the self-developed antigens and self-manufactured
antibodies, Norman's products features minimized intra-and inter- batch difference.
R&D shapes future, and concentration determines success. Driven by the commitment
to "provide complete solution to improve human health.Norman will consistently do its
utmost to drive the IVD industry forward and contribute more to human health.

Agent Wanted
If you are interested in working with us, please feel free to contact.

OEM will be highly welcome!
Welcome to our website!