


|
Nanjing Norman Biological Technology Co., Ltd
|
| Payment Terms: | T/T,L/C,D/A,D/P,WU,Paypal,Money Gram |
| Place of Origin: | Jiangsu, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Quantitative In Vitro Diagnostic Rapid
Test Kits For PCT (serum)
Methodology
Fluorescence Immunoassay
Intended use
Procalcitonin(PCT),also known as the Pre calcitonin, is composed of 116 amino acids.
Its molecular weight approximately 12.7kD. PCT is expressed by neuroendocrine
cells(includingC cells of the thyroid gland, lung and pancreas), and is decomposed
into three particles of thyrocalcitonin (immature), carboxyl terminal peptide and
amino terminal peptide by enzyme digestion.
Only a small amount of PCT is contained in the blood of healthy people.
PCT will be significantly increased after bacterial infection.
PCT is a specific marker of inflammatory disorder with bacterial infection and a
significant indicator of SIRS and sepsis caused by bacterial infection, especially for
severe sepsis and septic shock sepsis.
PCT plays an important role in differentiating inflammatory disorder caused by
infection and non-infection (such as autoimmune inflammation and acute rejection
after transplantation).
Meanwhile, PCT detection is of great significance in guiding the rational use of
antibacterials,evaluating therapeutic effect and shorten the using time of
antibacterials.
Fluorescence Immunoassay Analyzer

Fluorescent Immunoassays (FIA)
Fluorescent Immunoassays are simply a different type of immunoassay,
The key variable is the biochemical technique used for detecting the binding
of the "detection" antibody and the analyte molecule.
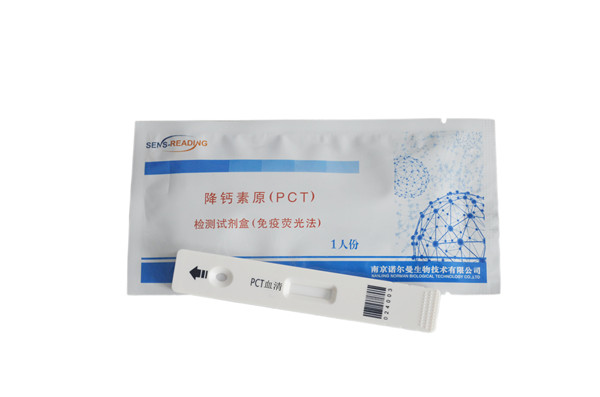
PCT Serum
1.Convenient and accurate solution of bacterial infection;
2.Evaluation indicator of infection degree;
3.Guidance of antibiotics use.
Test Method
1.Please read the manual and instrument operation carefully before using.
2.Check whether the equipment can operate regularly, and prepare other related materials.
3. Allow the test device, PCT buffer and specimen balanced to room temperature (15-30℃) prior to testing.
4.Minimizing exposure to damp air.
Test Procedure
1.Open the Immune-fluorescence quantitative analyzer.
2.Check whether the batch number of IC card and reagent box is consistent, and then insert
IC card into the instrument to get the curve we wanted.
3.Remove the test device from the protective pouch and place it on a flat surface. Draw in
30ul of whole blood sample to the centrifugal tube, and dilute with 90ul of PCT whole blood
buffer. After gently mix the sample, add 90ul of diluted whole blood sample into the sample
well. Place the test strip at room temperature for 15 minutes.
4.Insert the test card into the hole of immune-fluorescence quantitative analyzer, and click
start, the instrument will scan the test card automatically.
5.Immune-fluorescence quantitative analyzer detects results automatically and calculates
the content of PCT in the sample.
6.Specimens, used strip and transfer pipettes may be potentially.
Interpretation of test results
1.The analysis of test results are accomplished by immune-fluorescence quantitative
analyzer of our company's NORMAN series;
2.The concentration of PCT in the unknown samples can be read directly in the
corresponding batch of ID card standard curve by fluorescence signal value we measured.
The default results use ng/ml as a unit.
Expected values/Reference range
1.The reference range study was conducted based on PCT content of 95% of the
distribution range of statistical analysis in 120 healthy people, the result was as follows:
2.Normal reference values:<0.05ng/ml;
3.It is recommended that each laboratory established its own reference range, which may
be unique to the population it serves depending upon geographical, patient, dietary, or
environmental factors.
Notice of detection result
1.The test results should take clinical symptoms,health history,other laboratory tests, etc.
into account,just for clinical reference,nor as a sole factor of clinical diagnostic;
2.The concentrations of PCT in body as followed patients are higher than normal, but not as
the main basis for its determination of systemic infection.For instance,the first day after the
trauma, major surgery, burns, treatment with OKT3 antibodies, other drugs to stimulate
the release of inflammatory mediators cell, small cell lung cancer, medullary thyroid C cell
carcinoma; infants born within 48 hours;there are long-term or severe patients with
cardiogenic shock, and long-term patients with severe organ perfusion abnormalities, etc;
3.All operation must be strictly in accordance with operating procedures to get the correct
result. The accuracy of test results may be affected by any changes to operational
procedures;
4.Possible causes of false positive results: heterophilic antibodies;some non-specific blood
components which have similar epitopes to capture the fluorescent labeled antibody.
PCT serum show



Package & Delivery

About us
Nanjing Norman Biological Technology Co., Ltd is dedicated to R&D and manufacturing of
automated chemiluminescence system. Founded in 2008, Norman biological has been
upholding the idea that R&D shapes future ,and concentration determines success. Ever
since the beginning,Norman has been focusing on developing and manufacturing
chemiluminescence and immunofluorescence reagents.
Norman's manufacture center is located in the Yuhua District and owns an over 2,000m2
GMP-approved clean workshop. The R&D base, which is over 2,000m2 , is located in
state-level new Jiangbei district. Now there are over 100 R&D engineers, 40% of which
hold a PhD or master's degree.

Powered by advanced technology and excellent talents in the IVD field, Norman has been
consistently improving its innovation platform , and increasing R&D investment. Self-
innovation, combined with long-term strategic cooperation with universities and research
institutes and with outsourced technologies, ensures consistent improvement on product
quality. Norman's R&D field has covered instruments, reagents, and raw materials, and has
been entrusted by the Nanjing government to build a R&D center specialized in biological
chemistry and immunity diagnosis. Up to now, Norman has acquired over 20 patents.
Being an expert in automated chemiluminescence analysis , Norman owns independent
and completed intellectual property rights, and its products provide top-notch sensitivity,
precision and accuracy . Thanks to the self-developed antigens and self-manufactured
antibodies, Norman's products features minimized intra-and inter- batch difference.
After 8 years development, Norman is now on the fast track. An over 30,000m2 global
R&D center is in construction, and will hold more than 1,000 R&D engineers in the future.
R&D shapes future, and concentration determines success. Driven by the commitment to
"provide complete solution to improve human health", Norman will consistently do its
utmost to drive the IVD industry forward and contribute more to human health.
Certifications

Related Products
Agent Wanted
If you are interested in working with us, please feel free to contact.

Why us
Manufacturer and Exporter for nearly 10 years with self R&D Research Center;
Professional After-Sale service with On-site support worldwide;
CE/ISO13485 Certifications
OEM and ODM are available !